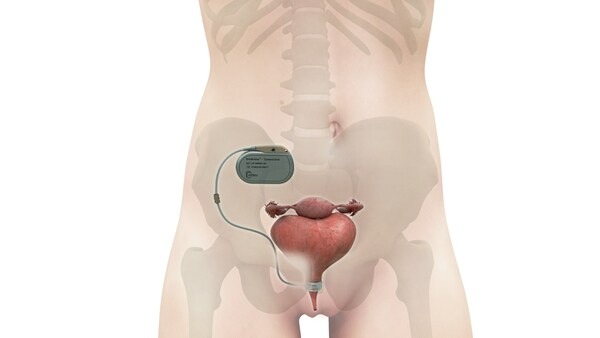
UroMems, a trailblazing company specializing in advanced mechatronic technologies for treating stress urinary incontinence (SUI), has achieved a significant breakthrough. The company revealed that it has successfully carried out the first-ever implant of the UroActive™ smart, automated artificial urinary sphincter (AUS) in a female patient. This groundbreaking procedure marks a new dawn for millions of women globally dealing with SUI. The insights gathered from this clinical study will play an instrumental role in shaping UroMems’ upcoming pivotal clinical trial across Europe and the U.S.
Hamid Lamraoui, UroMems CEO and co-founder, conveyed the significance of this development, “This is a landmark accomplishment for UroMems and women dealing with SUI. We aim to address the urgent need recognized by numerous physicians for an improved treatment method for this demographic.”
The first-ever implant procedure was successfully executed at La Pitié-Salpêtrière University Hospital in Paris, France by a skilled team consisting of Professor Emmanuel Chartier-Kastler, Dr. Aurélien Beaugerie, and Dr. Christophe Vaessen. This unprecedented procedure received approval from the National Agency for the Safety of Medicines and Health Products (ANSM), the French counterpart of the U.S. Food and Drug Administration.
The first recipient of the UroActive device exclaimed, “It’s magic. It’s a blessing to be able to carry out routine tasks that were previously impossible.”
The innovative UroActive is a smart, active implant designed to treat SUI, functioning on a MyoElectroMechanical System (MEMS). Positioned around the urethral duct, this device adjusts to the patient’s activity levels, offering ease of use and an enhanced quality of life compared to traditional options. The inaugural UroActive implant in a male patient was announced in November 2022. More recently, the U.S. FDA awarded UroMems the Safer Technologies Program (STeP) designation for UroActive, an initiative designed to expedite the development and marketing authorization for eligible devices.
Professor Pierre Mozer, UroMems CMO and co-founder, spoke on the matter, “The current state of treatment for women’s stress incontinence is simply not sufficient. Our ultimate goal is to make UroActive the preferred treatment for both physicians and patients.”
Stress urinary incontinence, characterized by involuntary urinary leakage, affects an estimated 130 million people across America and Europe. It occurs when the bladder pressure surpasses the pressure of the sphincter muscle around the urethra during high intra-abdominal pressure activities like coughing and exercising. Despite its significant impact on quality of life, leading to depression, low self-esteem, and social stigma, only two options have been available historically for moderate to severe SUI: a mesh sling or a manually-operated AUS.
Through the innovative application of embedded systems and micro-technologies in its smart active implants, UroMems aims to revolutionize the treatment of SUI. The company is committed to altering the perception that these disorders are simply inevitable aspects of aging without a definitive solution.