In a significant advancement for atopic dermatitis treatment, Sanofi Healthcare India Pvt. Ltd. has received approval for Dupixent® (dupilumab). This novel biologic medicine is the first of its kind to be approved for adults with moderate-to-severe atopic dermatitis whose condition is not adequately managed with topical therapies.
The key to Dupixent®’s success lies in its unique mechanism of action. Unlike conventional treatments that broadly suppress the immune system, Dupixent® targets the type 2 inflammation, the primary cause of atopic dermatitis, offering a specific and effective approach to treatment.
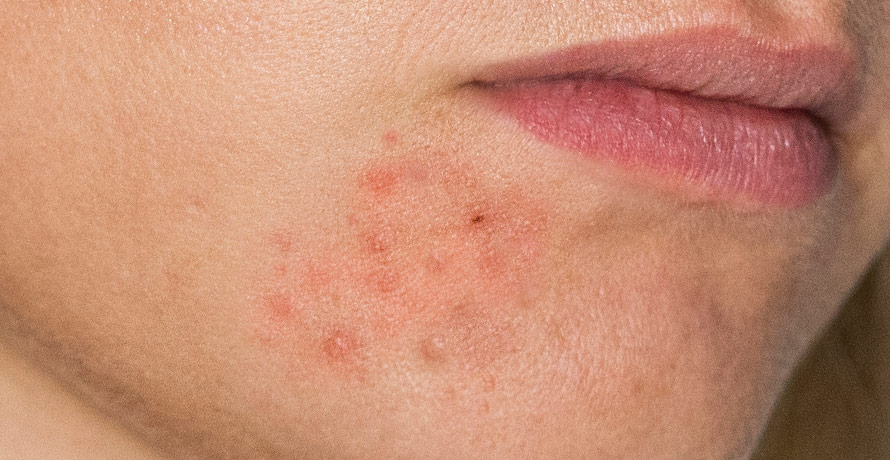
Atopic dermatitis, a chronic type 2 inflammatory disease, often presents as a skin rash. The disease’s moderate-to-severe form often leads to intense itching, skin dryness, redness, and oozing, significantly impacting patients’ quality of life.
Dupixent® has already shown considerable success in over 60 countries, including the U.S., the European Union, and Japan, bringing significant relief to patients with this difficult-to-treat condition. This approval brings a glimmer of hope to the Indian patients who have been struggling to find effective treatments for this persistent skin disease.
Resulting from a global collaboration between Sanofi and Regeneron, Dupixent® has already been administered to over 600,000 patients globally, showing promise in delivering a significant improvement in patients’ quality of life. The arrival of Dupixent® in India is set to transform the landscape of atopic dermatitis treatment for the better.